Pharmaceutical Reverse Osmosis Pure Water Equipment
Product Description
Product Description
Ultra-pure water equipment adopts pretreatment, reverse osmosis technology, ultra-purification treatment and post-treatment methods to almost completely remove the conductive medium in the water, and remove the non-dissociated colloidal substances, gases and organic substances in the water to a very low level. Water treatment equipment is a choice because Pure Water Making Equipment has high efficient technology .
Pure water equipment, it uses mainly reverse osmosis Membrane technology. Its working principle is to apply a certain pressure to the water to make the water molecules and ionic mineral elements pass through the reverse osmosis membrane, and most of the inorganic salts (including heavy metals), organic matter, bacteria and viruses dissolved in the water are impenetrable. Pass the reverse osmosis membrane, so that the pure water that has been permeated and the concentrated water that cannot be permeated are strictly separated; the pore size on the reverse osmosis membrane is only 0.0001 microns, while the diameter of the virus is generally 0.02-0.4 microns, and the diameter of ordinary bacteria is 0.4 -1 micron.

1. Different uses
(1) Purpose of Ultra-Pure Water Equipment:1. Production and cleaning of ultrapure materials and ultrapure reagents. 2. Production and cleaning of electronic products. 3. Production of battery products. 4. Production and cleaning of semiconductor products. 5. Production and cleaning of circuit boards. 6. Production of Other high-tech fine products.
(2) Purpose of pure water equipment:
1. Chemical water treatment in power plants 2. Ultra-pure water in the electronics, semiconductor, and precision machinery industries 3. Preparation of food, beverages, and drinking water 4. Small pure water stations, drinking pure water for groups 5. Fine chemicals, water for sophisticated subjects 6. Preparation of high-purity water required by other industries 7. Process water for pharmaceutical industry 8. Desalination of sea water and brackish water
Second, the conductivity of water is different
1. Water quality of ultrapure water: Resistivity>15MΩ.cm Ultrapure water quality is divided into five industry standards, namely 18MΩ.cm, 15MΩ.cm, 10MΩ.cm, 2MΩ.cm, 0.5MΩ.cm. Distinguish different water quality.
2. Pure water: industrial pure water and drinking pure water, iPharmaceutical water treatment reverse osmosis pure water equipment, if the raw water quality is relatively good, for example, the conductivity of the raw water quality in the south is below 100μS/cm, the pharmacopoeia standards do not have much restriction on the water production process, and single-stage reverse osmosis equipment can be used. Can meet customer's water demand. If the customer has higher water quality requirements and needs to meet the US Pharmacopoeia standard, it is recommended to use the EDI process, because the conductivity of the EDI process water is lower than that of the RO water. Generally, the raw water in the north has a relatively high salt content, and an EDI device can be added after the secondary reverse osmosis device to achieve the purpose of deep desalination. Pharmaceutical factories have many requirements for purified water. How many levels of reverse osmosis are used in water treatment reverse osmosis pure water equipment needs to be combined with the actual situation of the customer to select the appropriate process, so as to rationalize the investment and operating costs and ensure the stable and reliable water production of the system. .

01
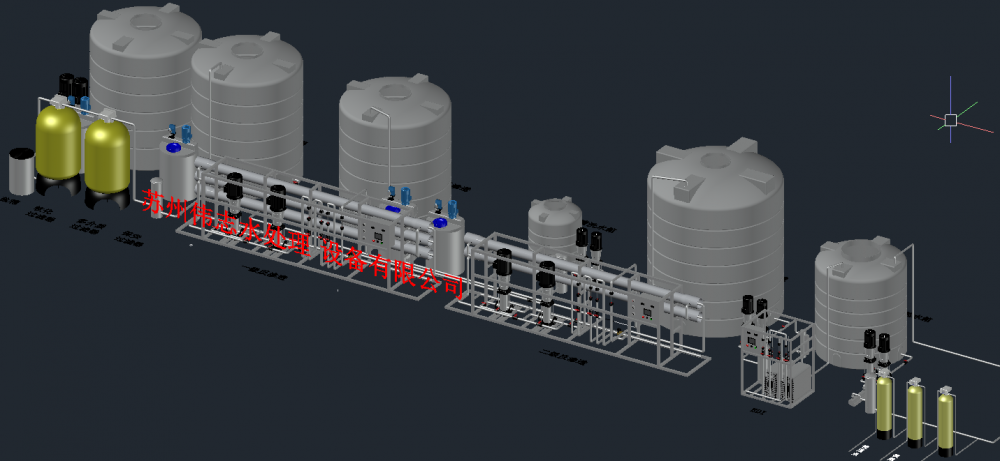
The system design process is:Raw water pump-mechanical filter-activated carbon filter-softener-microfiltration-high Pressure Pump-primary reverse osmosis-intermediate Water Tank-secondary reverse osmosis-pure water tank-pure water pump-ultraviolet sterilization-fine filter
System configuration description:
The system is all made of 304 and 316 stainless steel, PLC control and fully automatic operation.
02
Use characteristics of pure water equipment:
1. It will not corrode the pure water equipment and can extend the service life of the equipment.
2. The equipment does not change the chemical properties of water and has no other effect on the human body.
3. The pure water equipment is small in size, easy to install and use, and can be used unattended for a long time.
4. After the water flows through the equipment, it can become magnetized water. Pure water equipment can suppress and kill microorganisms in the water to a certain extent.
5. The equipment has the function of descaling. The equipment is installed in the water circulation system, and the treated scale is granular, which can be discharged with the sewage pipe without blocking the pipe system. After the old scale falls off, no new scale will be produced within a certain range.

The following seven points should be considered in the selection of reverse osmosis pure water equipment for water treatment in pharmaceutical plants:
1. Raw water quality
The raw water quality report and the water demand table are the key basis for the design of purified water. From the water quality report, you can learn the key indicators of the purified water system design, such as electrical conductivity, microbial limit, bacterial endotoxin, pH value, etc.
2. Water quality and regulations
The pharmacopoeias of various countries have clear requirements for the various indicators of purified water, and the legal standards that need to be clearly followed when designing the system. For example, the purified water is designed in accordance with the water quality standards of the Chinese, American and European Pharmacopoeias, and meets the certification requirements of GMP, FDA, cGMP, ISPE , FDA guidelines, ASTMD1193-2011, ISO13485 medical device quality system certification, EU CE certification.
3. Water production and system recovery rate
When designing the system, it is necessary to count the water production, understand the water demand of the enterprise, and provide personalized water system solutions based on URS. In terms of system recovery rate, the secondary RO concentrated water and EDI concentrated water are usually returned to the original water pipe and recycled to save water resources and reduce operating costs.
04
4. Microbial inhibition
The number of microorganisms is one of the important indicators to measure the quality of purified water. Excessive microorganisms will cause the water quality to fail. A set of qualified purified water preparation system inhibits the growth of microorganisms in terms of surface roughness, pipeline slope, pipeline flow rate, dead angle 3D design, and air blocking. In addition, considering the risk of microbial contamination caused by stagnant water residues, Valves that come into contact with purified water should choose a diaphragm Valve instead of a ball valve. The online instrument should adopt the diaphragm type, clamp connection, not only the dead angle is short, but also easy to disassemble and assemble.
5. Process selection and safety
According to the raw water quality report and product water quality requirements, a reasonable process is used to prepare purified water. The process flow of purified water mainly includes pretreatment + primary reverse osmosis (RO) + secondary reverse osmosis (RO); pretreatment + primary reverse osmosis (RO) + electrodeionization (EDI); pretreatment + primary reverse Permeation (RO) + secondary reverse osmosis (RO) + electrodeionization (EDI).
6. Disinfection method
The commonly used disinfection methods for purified water systems are pasteurization, ultraviolet disinfection and ozone disinfection. Among them, pasteurization heats the circulating purified water to 80°C and maintains it for a period of time to effectively prevent the growth of microorganisms in the distribution system; ultraviolet disinfection affects the formation of biofilms by slowing down the growth of new colonies in the system and kills the water Free microorganisms extend the disinfection cycle of the distribution system; ozone disinfection uses ozone as a spectral bactericide to kill bacterial propagules and spores, viruses, fungi, etc., and can destroy botulinum toxin. In addition to the advantages of simple operation, no fluctuations in water temperature, short disinfection time, and biofilm degradation, this method has a large choice of system piping. Which disinfection method to choose depends on the actual situation of the enterprise.

Product Categories : Pure Water Equipment > First-level Reverse Osmosis Pure Water Equipment









